Operating Theatre Ventilation ISO 5 for orthopedic surgery ( orthopedic O.R.)
The operio mobile laminar air flow system makes it possible to upgrade existing operating theatres from ISO 7 to ISO 5
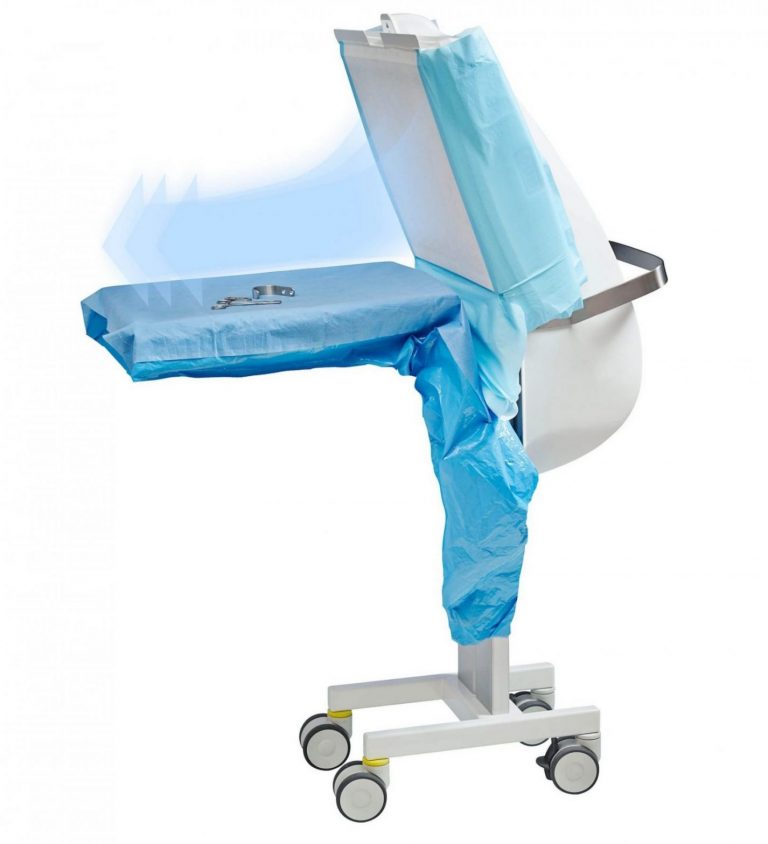
Portable laminar air flow units with integrated sterile air flow via Hepa H 14 filter
The focus-oriented, mobile laminar air flow devices generate an ultraclean, germ-free air flow by filtering the air with a Hepa H14 filter, which can be aimed precisely at the desired operating field area. The “sterile air flow” is not hindered neither by the operating theater lights nor by the operating theater team. In this way, extremely low-germ air can be created in the area of the operating theater and the instruments, which makes it possible to operate under perfectly hygienic conditions.
The mobility of the devices and the inexpensive price makes it possible to have high aseptic conditions in every existing operating theater.
Periprosthetic joint infection (PJI) is currently the leading cause of failure for primary and revision total knee (TKA) and total hip arthroplastyy. Every surgeon opens implants only at the very last minute before implantation to keep them sterile in order to avoid infections – the necessary surgical instruments, on the other hand, are left unprotected on the instrument table for a long time, where they become contaminated and might cause an infection.
The actual source of the bacteria is not in the air, but in the people in the operating theatre, who are the origin of the fatal pathogens that arise during implant insertion. Humans can shed hundreds to thousands of bacteria-bearing particles per minute (Bethune, 1975; Whyte et al., 1976; Whyte et al., 1978), which can settle onto surfaces in the operating theatre environment and especially on instruments. It can be assumed that 90% of early perioperative infections of joint prostheses are caused by microbial entry during surgery, whereby the entry of microorganisms into the wound is caused either by direct contact or by aerogenic contamination. (Uckay et al. (2009).
The most important measure against infections is to consistently maintain the highest hygiene standards during surgery especially during the implantation of implants. To minimise the intraoperative transmission of bacteria, laminar airflow ceilings are used in many hospitals to fend off bacteria. However, in the vast majority of operating theatres the existing supply-air ceilings are far too small to also cover the instrument preparation area. A sterile operating theatre does not exist- there are only sterile zones in the O.R. directly below the air outlet.
Various studies have made it very clear that instruments in an conventional operating room ( ISO 7) and instruments prepared outside the air supply ceiling of a laminar air flow system (ISO 5) will become contaminated after a short time. The longer the operation lasts, the higher will be the risk of instrument contamination and thus of infection.(Thomas Benen1, Frank Wille1*, Lüder Clausdorff2, The influence of different ventilation systems on microbiological instrument cleanliness Hyg Med 2013; 38-4).
Portable laminar air flow units with integrated sterile air flow via Hepa H 14 filter
The Steristay Instrument Table and Operio Mobile Laminar Air Flow unit filter the ambient air through a highly purifying 14 Hepa filter with an efficiency of over 99.995%, which eliminates bacteria and micro-organisms (including coronavirus covid 19) by over 99.9%!
The Operio unit and Steristay Instrument Table ensure that instruments and implants remain sterile both during preparation and throughout the surgical procedure.
Periprosthetic infections of the hip or knee joints
Periprosthetic infection (PPI) after an endoprosthetic joint replacement is a serious complication. Treating such infections requires a great deal of financial, personnel and time resources, which is beyond the scope of many clinics. Periprosthetic infection (PPI) after an endoprosthetic joint replacement is a serious complication. In the case of revision arthroplasty, the percentage of periprosthetic infections can be as much as 4%. (Perka C, Haas N: Periprosthetic infection. Chirurg 2011; 82:218–26)
In the USA, for a single episode of care, the direct cost of treating prosthetic joint infection (PJI) has been estimated as approximately US$ 100,000 ( Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg. 2005;87(8):1746–51.) with the overall lifetime treatment cost for a 65-year-old estimated at US$ 390,806 (Parisi TJ, Konopka JF, Bedair HS. What is the long-term economic societal effect of periprosthetic infections after THA? A Markov analysis. Clin Orthop Relat Res. 2017;475(7):1891–900)
Traceability for defensive medicine
The system is equipped with traceability for defensive medicine: barcode and label on the cover sterile housing can prove that a device compliant with Standard ISO5 has been used, at any time.
Upgrade ISO 7 operating theatres – extend the sterile zone in the ISO 5 operating theatre
The Sterile Air Flow units enable upgrading an existing ISO 7 operating theatre to ISO 5 or extending the sterile zone in an existing operating theatre in order to prepare the instruments and implants under sterile conditions and to guarantee the best aseptic conditions during the operation.
The Norwegian case study.
The two Operio and SteriStay mobile units have been used for the past 3 years at the Drammen Hospital, Norway. Employed to complement the standard structural ventilation system, their purpose is reducing the quantity of airborne bacteria known to be a risk factor for open wounds and sterile instruments.
Before using Operio and steristay, the infection risk was 1.5-2% – a common infection rate, as reported in Scandinavian databases.
Infection statistics on 5,000 surgeries show that, for knee and hip replacement surgeries, the risk of deep infections has been reduced to 0.27%.
This data conclusion supports the idea that reducing the intraoperative contamination is essential to prevent deep infections, especially in those procedures known to be subject to them.

Asepsis in Complex Traumatology.
Many companies that produce osteosynthesis devices, still provide implant materials in boxes, and not in sterile packaging.
In complex traumatology surgery, it is possible that plates and screws are opened at the beginning of the procedure, while preparing the instruments, and kept without protection until the end of the surgery.
A pointlessly dangerous exposure that may last even several hours.

Benefits:
Prevention of periprosthetic infections
Instruments and implants can be prepared under aseptic cleanroom conditions
Enlarge of the protective zone in the operating theatre without turbulence
The Sterile Air Flow is experienced as very pleasant by personnel, because the air it produces is not cold and it is also very quiet – comparable to a projector
to upgrade existing operating and minor procedures rooms quickly and cost-effectively.
The units are extremely small and easy to handle
tracking systems for defensive medicine
Related products
Operio portable laminar air flow
TOUL Operio focused laminar air flow is a device that can be used in any environment: from operating rooms to clinics.
Go to product
Steristay instrument table with hepa filter
TOUL Steristay– Focused laminar flow with built-in backtable is ideal to keep the surgical instruments sterile.
Go to product
 it
it en
en fr
fr de
de

















